Cystic fibrosis (CF) is one of the most common genetic disorders, with a carrier frequency of 4% (1 in 25) in the Greek population.
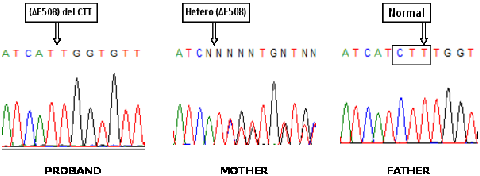
The number of disease mutations is high (> 1500), but a specific mutation, F508del, is present in approximately 53% of Greek patients and has a carrier frequency of about 2% (1/50) in the Greek population.
Therefore, by detecting the mutation we will reveal about 50% of carriers and in such a case it is important to proceed directly to genetic testing of the father (for at least 85% of Greek CF mutations), to determine whether it is necessary to perform prenatal diagnosis of the fetus early in pregnancy.
Therefore, this particularly useful test affords the detection of a large percentage of carriers for the disease and will reveal couples at-risk, early in pregnancy (see our relevant publication: Konialis C. et al, Fetal Diagn Ther. 2007; 22(1):41-4).
Note: This test does not replace carrier detection for CF – Cystic Fibrosis – 85% of mutations in the Greek population
Pre-lingual, non-syndromic hearing loss, i.e. the type that occurs before the development of speech development in children, in >50% of cases is due to a genetic disease, which is mainly caused by recessive mutations (inherited from carrier parents).
There are more than 100 genes involved in hearing loss. However, mutations in only one of the genes, the GJB2 gene (connexin 26), represent more than 65% of non-syndromic hearing loss cases.
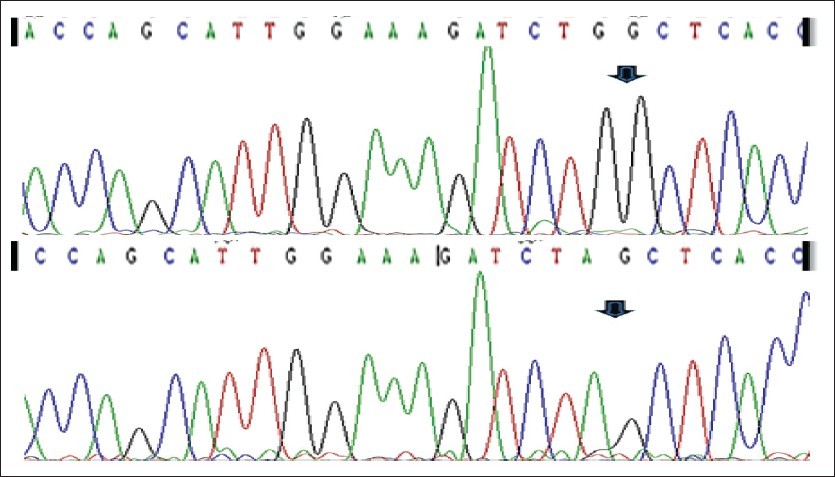
In fact, the 35delG mutation of the GJB2 gene (connexin 26) accounts for ~90% of all mutations of the disease and has a carrier frequency of approximately 3.5% in the general population (1 in 28).
In case the presence of the 35delG mutation is detected in the maternal blood sample, an immediate and comprehensive mutation analysis of this disorder from a paternal blood sample is necessary, in order to evaluate the risk for the fetus. This particularly useful test affords the detection of a large percentage of carriers for the disorder and will reveal couples at-risk, early in pregnancy.
Note: This test does not replace carrier detection for Non-syndromic hearing loss and deafness (DFNB1-GJB2, GJB6).
The detection of the F508del and 35delG mutations is performed from a maternal dry-blood sample and may be performed in parallel with the risk for Down syndrome using biochemical markers from a maternal dry blood sample in the 1st or 2nd trimester.
Samples should be collected using the special cards provided. Instructions for sampling procedures are printed at the back of the card.
After the blood sample has completely dried up, it may be stored dry in an envelope, at room temperature. For longer periods and during the warm months of the year, it is advised to store the sample at 4°C. Shipment should be made by mail or courier service.
It is important to state that the sampling method used, through a maternal dry blood sample on the special filter paper, is an internationally accepted and scientifically fully documented method. It facilitates sampling and guarantees the stability of the biochemical markers in the sample and therefore the accuracy of measurement, and is applied by several large and prestigious laboratories worldwide.
Please note: that both detections of the F508del mutation as well as the 35delG mutation, does NOT CONSTITUTE a full mutation analysis for cystic fibrosis or a full mutation for autosomal recessive hearing loss.